Multiple Choice
What change in reaction direction occurs if dilute HCl is added to a H2PO4 − solution?
H2PO4 1− + H2O 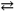 HPO42 − + H3O+
HPO42 − + H3O+
A) shifts to the right
B) shifts to the left
C) no change in the reaction
D) there is insufficient information to solve this problem
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: The term "strong acid" refers to any
Q2: What is the pH of a 0.150
Q4: If a reaction is in a state
Q5: Which of the following is a weak
Q6: According to the Brønsted-Lowry theory of acids
Q7: The synthesis of the pesticide thionyl chloride
Q8: Consider the weak base ClO<sup> − </sup>.<br>ClO<sup>
Q9: A catalyst will increase the rate of
Q10: A large "K" value (>0) indicates that
Q11: If NaCl is added to a saturated