Multiple Choice
Identify the correct balanced chemical equation for the cell: 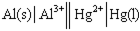
A) Al( s ) + Hg2+( aq ) → Al3+( aq ) + Hg( l )
B) 2 Al( s ) + Hg2+( aq ) → 2 Al3+( aq ) + Hg( l )
C) Al( s ) + 3 Hg2+( aq ) → Al3+( aq ) + 3 Hg( l )
D) 2 Al( s ) + 3 Hg2+( aq ) → 2 Al3+( aq ) + 3 Hg( l )
Correct Answer:

Verified
Correct Answer:
Verified
Q30: The Nernst equation describes the pH required
Q31: The species undergoing reduction is referred to
Q32: Single use, non-rechargeable batteries are referred to
Q33: How long will it take to collect
Q34: The kinetics of uniform corrosion will speed
Q35: The SHE is assigned a voltage of
Q36: What is the E<sup>0</sup> value for the
Q37: In balancing electrochemical half-reactions in acidic media,
Q39: The salt bridge between 1/2 reactions maintains
Q40: What is the oxidation number of Cl