Multiple Choice
What is the E0 value for the Galvanic cell formed from these two half-reactions?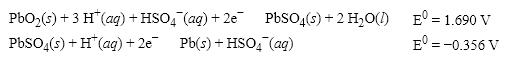
A) +1.334 V
B) − 2.046 V
C) +2.046 V
D) +2.758 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: In the formation of iron (II) oxide,
Q2: In the decomposition of hydrogen peroxide, which
Q3: Galvanic corrosion may occur only when two
Q4: In a redox reaction:<br>A) there is a
Q5: Which phrase best describes the half-reaction as
Q7: In the formation of iron (II) oxide,
Q8: Species with positive standard reduction potentials are
Q9: Balance the following electrochemical reaction in acid:<br>MnO<sub>4</sub><sup>
Q10: What is the E<sup>0</sup> value for the
Q11: Secondary cells are designed to be rechargeable.