Multiple Choice
Which of the following acids would serve as a good buffer for a reaction at pH = 8.0? 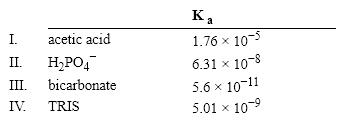
A) I
B) II
C) III
D) IV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: The water molecule is polar because:<br>A) Electrons
Q5: Exhibit 2B Contains information on the pK's
Q6: If a solution has a pH =
Q7: Distinguish between the hydrogen bonding found
Q8: Which of the following is true regarding
Q10: Exhibit 2B Contains information on the pK's
Q11: Which of the following is true?<br>A) The
Q12: True hydrogen bonds can NOT form between
Q13: The non-covalent interaction below associated with the
Q14: Which of the following equations represents the