Multiple Choice
If the pH of 1 liter of a 1.0 M carbonate buffer is 7.0, what is actual number of moles of H2CO3 and HCO3 − ? (pK = 6.37) 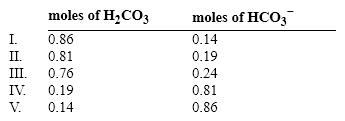
A) I
B) II
C) III
D) IV
E) V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q55: Which of the following elements has the
Q56: Using the Henderson-Hasselbalch equation, calculate the pH
Q57: In a titration of a weak acid
Q58: Calculate the final pH of a solution
Q59: Molecules which contain both hydrophilic and hydrophobic
Q61: When does a weak acid buffer best?<br>A)
Q62: Buffering capacity refers to<br>A) the effectiveness of
Q63: The tendency for an atom to attract
Q64: The substance most likely to form a
Q65: Which of the following statements is