Multiple Choice
Exhibit 2B Contains information on the pK's of some common buffers. 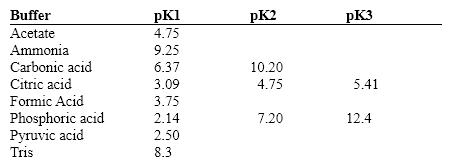 Refer to Exhibit 2B. A carbonate buffer would work well at this pH:
Refer to Exhibit 2B. A carbonate buffer would work well at this pH:
A) 4.0
B) 6.0
C) 8.0
D) 10.0
E) 6.0 and 10.0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q47: Exhibit 2A The structure of ATP with
Q48: The inflection point of the titration curve
Q49: Bases are<br>A) proton donors.<br>B) proton acceptors.<br>C) hydrogen
Q50: Which of the following is a true
Q51: A hydrogen bond is a special type
Q53: Exhibit 2B Contains information on the pK's
Q54: If atoms with greatly differing electronegativities form
Q55: Which of the following elements has the
Q56: Using the Henderson-Hasselbalch equation, calculate the pH
Q57: In a titration of a weak acid