Multiple Choice
Exhibit 2B Contains information on the pK's of some common buffers. 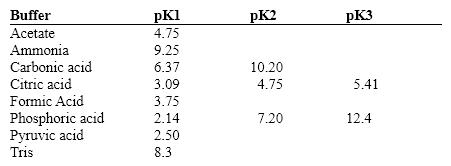 Refer to Exhibit 2B . Which of the following would make the best buffer at pH =10.0?
Refer to Exhibit 2B . Which of the following would make the best buffer at pH =10.0?
A) Acetic acid and sodium acetate
B) Tris and its acid form
C) H2CO3 and NaHCO3
D) Na2HPO4 and NaH2PO4
E) NaHCO3 and Na2CO3
Correct Answer:

Verified
Correct Answer:
Verified
Q16: Nonphysiological buffers such as HEPES and PIPES
Q17: Which of the following is true about
Q18: Which of the following is not considered
Q19: Explain why carbon dioxide is nonpolar.
Q20: Buffers which lack biological activity and are
Q22: Which has the greater pK<sub>a</sub>, a weak
Q23: Many of the properties of water can
Q24: How does the strength of hydrogen bonds
Q25: Which of the following principles states
Q26: A buffer solution<br>A) is used to control