Multiple Choice
For a general equation , the relation between the free-energy change ( ) for the reaction under any condition and the free energy change under standard conditions ( ) can be written as _____.
A) 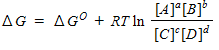
B) 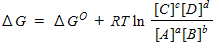
C) 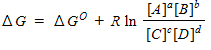
D) 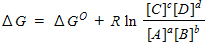
Correct Answer:

Verified
Correct Answer:
Verified
Q38: Consider this rxn which has a Δ
Q39: The oxidation of nutrients supplies the energy
Q40: The standard state usually used in biochemistry
Q41: Consider this rxn which has a Δ
Q42: "Metabolism" refers to<br>A) the breakdown of larger
Q44: In the coenzyme FAD the site to
Q45: Consider these reactions: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8676/.jpg" alt="Consider these
Q46: During reduction<br>A) electrons are lost.<br>B) electrons are
Q47: Consider this rxn which has a Δ
Q48: Which of the following statements apply to