Multiple Choice
Figure 7-1 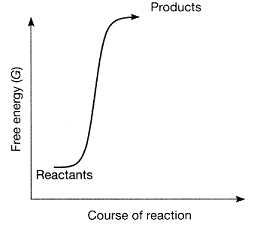 Which statement best describes the plot in the accompanying figure?
Which statement best describes the plot in the accompanying figure?
A) The figure represents an endergonic reaction.
B) The figure represents a spontaneous reaction.
C) The products have more free energy than the reactants.
D) The reactants have more free energy than the products.
E) The reaction is endergonic, and in addition, the products have more free energy than the reactants.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: A cell must expend energy to produce
Q9: Which of the following accurately represents the
Q13: Which of the following statements concerning ATP
Q15: As you climb a flight of stairs,
Q16: Figure 7-2 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8677/.jpg" alt="Figure 7-2
Q17: An enzyme binds its substrate at the
Q18: What is the ultimate source of energy
Q19: Consider the following two chemical equations:<br>A. glucose
Q44: In competitive inhibition, the inhibitor binds to
Q47: An endergonic reaction can proceed only if