Multiple Choice
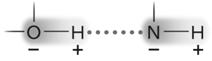
Figure 2.3
Answer the question using the accompanying figure. The molecule shown is held together by ____.
A) polar covalent bonds
B) van der Waals forces
C) ionic bonds
D) hydrogen bonds
E) nonpolar covalent bonds
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: In a molecule of methane, CH<sub>4</sub>, each
Q17: For each of the following situations, choose
Q17: Why is iodine considered a trace element,
Q27: In the presence of water, nonpolar associations
Q39: In contrast to ionic bonds, covalent bonds
Q62: High levels of carbon dioxide in the
Q64: The substance H<sub>2</sub>O is considered to be
Q65: Describe the difference between cohesion and adhesion,
Q72: The most common isotope of carbon has
Q81: Carbon dioxide is an element.