Multiple Choice
A neutral isotope of an element contains 21 electrons and 24 neutrons. What is the following representation for this nucleus?
A) 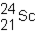
B) 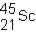
C) 
D) 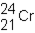
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q74: One mole of silver would contain the
Q75: Calculate the weight percentage of hydrogen in
Q76: How many moles of oxygen atoms are
Q77: Naturally occurring neon (Ne)has the following isotopic
Q78: Neutral isotopes of the same element have
Q80: Oxalic acid is found in many plants,
Q81: Naturally occurring lithium (Li)consists of only two
Q82: The most accurate way to determine atomic
Q83: The average relative mass of an ozone
Q84: Isotopes of the same element always have