Multiple Choice
Which of the following is the correct valence electron configuration of phosphorus?
A) 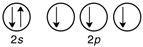
B) 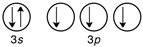
C) 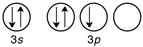
D) 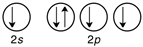
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q76: How does Schrödinger's quantum mechanical model
Q77: Silver, Ag, belongs to what period of
Q78: Which of the following is considered a
Q79: The fourth shell contains four subshells.
Q80: The electron configuration for each shell starts
Q82: Based on the physical states of the
Q83: Which of the following elements is found
Q84: One mole of an element would weigh
Q85: The total number of unpaired electrons in
Q86: Which of the following will not have