Multiple Choice
Which of the following correctly arranges 1.00 M solutions of the strong electrolytes in order of increasing boiling point (lowest to highest) ?
A) 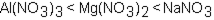
B) 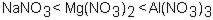
C) 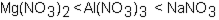
D) All have the same boiling point.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q65: Match the types of colloids with
Q66: One test to determine if a mixture
Q67: Attractive forces between solute and solvent molecules
Q68: All ionic compounds are soluble in water.
Q69: When a patient's blood electrolyte levels are
Q71: The solubility of a substance can be
Q72: Which of the following pass through both
Q73: The cleaning action of soaps and detergents
Q74: How many moles of Na<sub>2</sub>CO<sub>3</sub> are needed
Q75: Light scattering is an effective way to