Multiple Choice
A solution is prepared at 75 °C by dissolving 50.0 g of A in 100 g of water. When this solution is cooled to 15 °C what happens based on the following solubility plot for A. 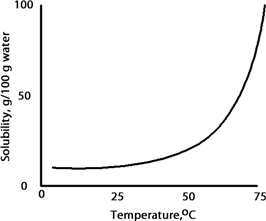
A) Solid A crystallizes and the solution is unsaturated.
B) Solid A dissolves and the solution is supersaturated.
C) Solid A crystallizes and the solution is saturated.
D) Solid A dissolves and the solution is unsaturated.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: Which of the following is not considered
Q16: Which compound is most soluble in a
Q17: The vapor pressure of a pure solvent
Q18: A solution is made by dissolving 15.0
Q19: A solution is made by dissolving 5.84
Q21: Which of the following pairs can produce
Q22: The primary intermolecular attractions between CH<sub>3</sub>-OH and
Q23: A colligative property<br>A)will only occur for aqueous
Q24: Identify a condition under which a
Q25: As NH<sub>4</sub>NO<sub>3</sub> dissolves in water, the resulting