Multiple Choice
What is the function of 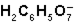 in the first ionization of citric acid?
in the first ionization of citric acid? 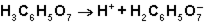
A) The ion serves as an Arrhenius acid in this reaction.
B) The ion serves as an Arrhenius base in this reaction.
C) The ion serves as the conjugate base of the acid, H3C6H5O7.
D) The ion serves as the conjugate acid of the base, H3C6H5O7.
Correct Answer:

Verified
Correct Answer:
Verified
Q82: A 25.00 mL sample of H<sub>2</sub>SO<sub>4</sub> acid
Q83: The goals of an acid\base titration is
Q84: When an inorganic acid and an inorganic
Q85: Which of the following is a weak
Q86: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8711/.jpg" alt=" is a stronger
Q88: HCl will react with<br>A)BaO<br>B)CaCO<sub>3</sub><br>C)Mg<br>D)All three are correct.
Q89: ? Identify the Brønsted bases in the
Q90: A 25.00 mL sample of hydrochloric acid
Q91: The term, strong acid, refers to<br>A)the number
Q92: The salt of a strong acid and