Multiple Choice
What are the missing products in the following reaction when it is written as a full equation? 2HBr + SrCO3 → ____ + ____ + H2O
A) SrBr2 + CO2
B) SrBr2 + 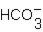
C) Sr2 + H2CO3
D) HBr + CO2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: The procedure commonly used to determine the
Q33: A Brønsted base is a proton donor.
Q34: When a strong acid is added to
Q35: Sodium nitrate in water will produce a
Q36: Gastroesophageal reflux disease (GERD)is the result of
Q38: The classification of an acid or base
Q39: Which of the following statements is true
Q40: Solutions with a pH less than 7.00
Q41: A solution has a pH of 8.72.
Q42: Nitric acid, HNO<sub>3</sub>, is a weak acid.