Multiple Choice
The expression for the K a of the weak acid HF would be which of the following?
A) 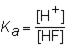
B) 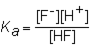
C) 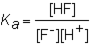
D) 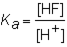
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: KOH is a strong base.
Q58: List the rules to be followed
Q59: NaCl is an example of a good
Q60: What volume of 6.0 M HNO<sub>3</sub> would
Q61: During a field trip to study the
Q63: The molar concentration of H<sup>+</sup> ions in
Q64: Consider the following list of p K
Q65: The pH of blood must remain stable
Q66: Identify the Brønsted acid(s)in the reaction. HIO<sub>3</sub>(aq)+
Q67: A patient comes to you suffering from