Multiple Choice
Which of these would be expected to have a trigonal planar electron geometry?
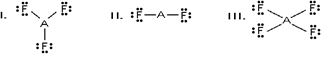
A) I and II
B) II and III
C) I, II, and III
D) I and III
E) I only
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: An atom with two valence electrons will
Q24: Which of the following molecules is nonpolar?<br>A)
Q25: Which of the following are polar molecules?<br>I.
Q26: Of the bonds below, the most polar
Q27: Which of these is the correct Lewis
Q29: Which of these molecules contains a triple
Q30: How many lone pairs of electrons are
Q31: List the elements Li, K, Al, Br,
Q32: Which of the following results in bent
Q33: Which of these are polar molecules with