Multiple Choice
Three sets of quantum numbers are given below. Choose the best answer.
I. n = 3, 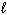 = 3,
= 3, 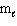 = 0, m s = 1 / 2
= 0, m s = 1 / 2
II. n = 2, 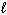 = 1,
= 1, 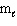 = 1, m s = 1 / 2
= 1, m s = 1 / 2
III. n = 4, 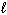 = 2,
= 2, 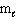 = 3, m s = - 1 / 2
= 3, m s = - 1 / 2
A) I and II are allowed sets, III is not
B) I and III are allowed sets, II is not
C) only I is allowed
D) only II is allowed
E) none of these are allowed
Correct Answer:

Verified
Correct Answer:
Verified
Q20: Calculate the frequency of light emitted when
Q21: The threshold frequency for the photoelectric effect
Q22: How many orbitals are present in a
Q23: How much energy does a single photon
Q24: Calculate the frequency of light whose wavelength
Q26: The energy of a photon of electromagnetic
Q27: Which orbital diagram for the ground state
Q28: How many electrons can be placed in
Q29: What is the maximum number of electrons
Q30: A subshell is described by assigning values