Multiple Choice
How many possible orientations are allowed when 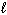 = 0?
= 0?
(s-type sublevel; How many possible 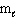 values are possible?)
values are possible?)
A) 1
B) 2
C) 3
D) 5
E) 7
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q47: What quantum number(s) is(are) required to completely
Q48: Which of the following atoms is paramagnetic
Q49: Which electronic configuration(s) listed below is(are) correct
Q50: What is the correct electronic configuration for
Q51: What is the energy of a photon
Q53: The Pauli exclusion principle:<br>A) allows only two
Q54: How many possible orientations are allowed when
Q55: An argon ion laser emits light at
Q56: What are the possible <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg" alt="What
Q57: What is the ground state electron configuration