Multiple Choice
Exhibit 11-2 The phase diagram below is needed for the following question(s) . 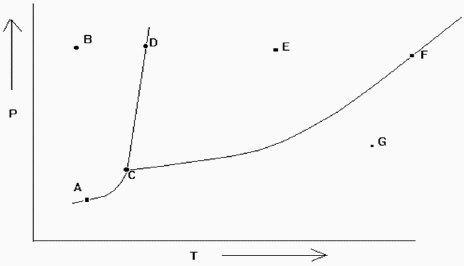
Refer to Exhibit 11-2. If one is at position A and the pressure is increased, which statement below is true?
A) One moves from a position of gas-liquid equilibrium to one in the liquid phase.
B) One moves from the gas phase to the liquid phase.
C) One moves from a position of gas-solid equilibrium to one in the liquid phase.
D) One moves from a position of gas-liquid equilibrium to one in the gas phase.
E) One moves from a position of gas-solid equilibrium to one in the solid phase.
Correct Answer:

Verified
Correct Answer:
Verified
Q88: Exhibit 11-2 The phase diagram below is
Q89: The basic repeating three-dimensional pattern of a
Q90: Exhibit 11-4 Consider Aluminum metal that crystallizes
Q91: At what angle are x-rays with a
Q92: A copper crystal is classified as:<br>A) ionic<br>B)
Q94: A crystal diffracts x-rays (£ = 154
Q95: For most substances, the enthalpy of the
Q96: On the basis of intermolecular forces of
Q97: Which crystal packing structure listed below provides
Q98: Which unit cell(s) provide(s) a coordination number