Multiple Choice
Exhibit 11-3 Consider the General Phase Diagram shown below to answer the following problem(s) . 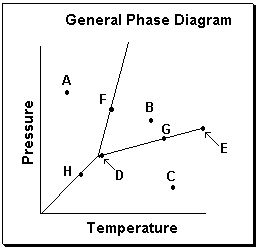
-Refer to Exhibit 11-3. Which temperature-pressure point on this diagram represents conditions in which the solid phase is present in equilibrium with the liquid phase?
A) A
B) B
C) C
D) F
E) G
Correct Answer:

Verified
Correct Answer:
Verified
Q44: What intermolecular force(s) of interaction is(are) present
Q45: Given the three statements below, pick the
Q46: An atom sitting in the center position
Q47: On the basis of intermolecular forces of
Q48: A metal that has a radius of
Q50: In a cubic array of atoms, how
Q51: Based upon an analysis of intermolecular forces
Q52: Which of the following substances have London
Q53: What intermolecular force(s) of interaction is(are) possible
Q54: What is the most important intermolecular force