Multiple Choice
What intermolecular force(s) of attraction is(are) present between two molecules of ethanol shown below?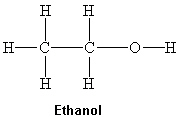
I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
A) I only
B) II only
C) III only
D) I and II
E) All of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: Exhibit 11-1 The heating curve below is
Q5: Exhibit 11-1 The heating curve below is
Q6: What intermolecular force(s) of attraction is(are) present
Q7: Which of the molecules shown below does
Q8: Which of the following molecules can form
Q10: Arrange the following molecules in order of
Q11: Exhibit 11-3 Consider the General Phase Diagram
Q12: The stacking of closest packing layers for
Q13: What type of crystal would CO<sub>2</sub> form
Q14: Gold crystallizes in a face-centered cubic array