Multiple Choice
For the reaction CN - (aq) + H₂ O ( 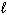 )
) 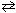 HCN (aq) + OH - (aq) , one of the acid-base conjugate pairs is:
HCN (aq) + OH - (aq) , one of the acid-base conjugate pairs is:
A) HCN, H2O
B) HCN, CN -
C) H2O, CN -
D) HCN, OH -
E) CN - , OH -
Correct Answer:

Verified
Correct Answer:
Verified
Q68: The pH of a vinegar solution is
Q69: Regarding acid strength, which of the following
Q70: Consider the salt pyridinium acetate, [(C<sub>5</sub>H<sub>5</sub>NH<sup>+</sup>)(C<sub>2</sub>H<sub>3</sub>O<sub>2</sub><sup>1 -
Q71: Which of the following substances would behave
Q72: What species are formed in the hydrolysis
Q74: What is the pH of a 0.10
Q75: After completing the following unfinished table, what
Q76: What is the pH of aqueous 0.10
Q77: What is the Calcium hydroxide concentration, [Ca(OH)<sub>2</sub>],
Q78: A weak monoprotic acid is dissolved in