Multiple Choice
In the acid-base equilibrium PO4 3 - + NH ₄+ 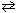 HPO3 2 - + NH₃, one conjugate acid-base pair is:
HPO3 2 - + NH₃, one conjugate acid-base pair is:
A) PO43 - , NH4+
B) NH4+ , HPO42 -
C) HPO42 - , NH3
D) NH4+ , NH3
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q121: What is the pH of a 0.25
Q122: Exhibit 15-3 Ephedrine (C<sub>10</sub>H<sub>15</sub>ON), a central nervous
Q123: Which solution is most basic, given K
Q124: What is the pH of a solution
Q125: Which of the following salts is considered
Q127: The hydronium ion concentration, [H<sub>3</sub>O<sup>+</sup>], for a
Q128: Select the best choice given the three
Q129: What is the pH of a 0.45
Q130: A 0.010 M solution of the strong
Q131: Exhibit 15-6 Consider an aqueous 0.25 M