Multiple Choice
In the reaction:
N₂H4 (aq) + H₂ O 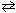 N₂H5 + (aq) + OH - (aq)
N₂H5 + (aq) + OH - (aq)
A) N2H4 and N2H5+ act as acids.
B) H2O and N2H5+ act as acids.
C) N2H5+ and OH - act as acids.
D) only OH - acts as a base.
E) none of these.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: After completing the following unfinished table, what
Q6: Which aqueous solution(s) below is(are) considered basic
Q7: At 37 ° C the autoionization constant
Q8: K <sub>b</sub> for ammonia is 1.8×10<sup> -
Q9: If the reaction below proceeds, which of
Q11: What is the pH of a 0.025
Q12: A 0.250 M aqueous solution of p
Q13: What is the acid ionization constant ,
Q14: What is the pH of an aqueous
Q15: 50 mL of 0.50 M NaOH is