Multiple Choice
Given the following Bronsted-Lowery acid-base reaction and its corresponding equilibrium constant, which substance is the strongest acid ?
CH ₃CH ₂ NH₃+ + ClO21 - 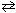 CH ₃CH ₂ NH₂ + HClO ₂ K = 2.1 10 - 9
CH ₃CH ₂ NH₂ + HClO ₂ K = 2.1 10 - 9
A) CH3CH2NH3+
B) ClO21 -
C) CH3CH2NH2
D) HClO2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: The pH of a certain specimen of
Q34: K <sub>a</sub> for HF is 3.5×10<sup> -
Q35: Which of the following is a property
Q36: To calculate the pH of a system
Q37: Exhibit 15-2 The pH of a solution
Q39: What is the hydroxide ion concentration in
Q40: Exhibit 15-5 Consider solving for the pH
Q41: Measurements show that the [OH<sup> - </sup>]
Q42: Consider the following unfinished table of weak
Q43: Exhibit 15-5 Consider solving for the pH