Multiple Choice
Aniline, C6 H5 NH₂ , is commonly used as a precursor for making dyes. Aniline is a base when dissolved in water. It ionizes as shown below. What is the base ionization constant, K b , of aniline if an aqueous 0.20 M C6 H5 NH₂ solution has a pOH value equal tO5 .05?
C6 H5 NH₂ + H₂ O 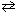 C6 H5 NH₃+ + OH -
C6 H5 NH₃+ + OH -
A) K b = 6.3×10 - 18
B) K b = 4.0×10 - 10
C) K b = 4.5×10 - 5
D) K b = 1.5×10 - 2
E) K b = 6.3×1010
Correct Answer:

Verified
Correct Answer:
Verified
Q12: A 0.250 M aqueous solution of p
Q13: What is the acid ionization constant ,
Q14: What is the pH of an aqueous
Q15: 50 mL of 0.50 M NaOH is
Q16: A nitric acid (HNO<sub>3</sub>) solution is 16.2
Q18: Which anionic base listed below would be
Q19: What is the pOH of a 1.0×10<sup>
Q20: An acid HB<sup>+</sup> has a K <sub>a</sub>
Q21: What is the acid ionization constant ,
Q22: Exhibit 15-6 Consider an aqueous 0.25 M