Multiple Choice
Exhibit 15-3 Ephedrine (C10H15ON) , a central nervous system stimulant, is used in nasal sprays as a decongestant. This compound is a weak organic base :
C10H15ON (aq) + H₂ O 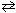 HC10H15ON + (aq) + OH - (aq) Consider a 0.035 M
HC10H15ON + (aq) + OH - (aq) Consider a 0.035 M
Solution of ephedrine that has a pH of 11.33 to answer the following problem(s) .
-Refer to Exhibit 15-3. What is the base ionization constant , K b , for the dissolution of ephedrine in water?
A) 6.3×10 - 22
B) 7.5×10 - 11
C) 1.3×10 - 4
D) 2.1×10 - 3
E) 6.4×10 - 2
Correct Answer:

Verified
Correct Answer:
Verified
Q82: The hydroxide ion concentration in a solution
Q83: What is the pH of a 2.6
Q84: The following equilibrium is established in water
Q85: What is the acid ionization constant ,
Q86: Pyridine (p K <sub>b</sub> = 8.74) reacts
Q88: Employing a neutralization reaction , which reactants
Q89: A weak acid<br>A) does not react with
Q90: Which of the following aqueous solutions is(are)
Q91: From the information given below which one
Q92: The definition of a Lewis base is:<br>A)