Multiple Choice
Exhibit 15-4 Consider dissolving NaF in water as shown in the equation below to answer the following question(s) :
NaF (s) + H₂ O ( 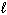 )
) 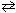 HF (aq) + NaOH (aq)
HF (aq) + NaOH (aq)
-Refer to Exhibit 15-4. What is the base ionization constant , K b , for this reaction?
K a (HF) = 6.8 10 - 4
A) K b = - 6.8×10 - 4
B) K b = 6.8×10 - 18
C) K b = 1.47×10 - 11
D) K b = 1.47×10 - 4
E) K b = 6.8×1010
Correct Answer:

Verified
Correct Answer:
Verified
Q106: The pH of a diet Coca Cola
Q107: Acid strengths are measured and compared how?<br>A)
Q108: Exhibit 15-4 Consider dissolving NaF in water
Q109: What species are formed in the hydrolysis
Q110: The weak acid HA dissociates with K
Q112: What is the pH of a solution
Q113: Which of the following salts is(are) considered
Q114: Which salt(s) listed below form(s) an acidic
Q115: Which substance in the reaction below behaves
Q116: A 0.20 M solution of the weak