Multiple Choice
Exhibit 15-4 Consider dissolving NaF in water as shown in the equation below to answer the following question(s) :
NaF (s) + H₂ O ( 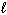 )
) 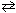 HF (aq) + NaOH (aq)
HF (aq) + NaOH (aq)
-Refer to Exhibit 15-4. What is the pH of this 0.100 M NaF solution?
A) pH = 2.08
B) pH = 5.91
C) pH = 8.08
D) pH = 11.83
E) pH = 11.92
Correct Answer:

Verified
Correct Answer:
Verified
Q60: Milk of magnesia is essentially a saturated
Q61: What is the pH of 3.9×10<sup> -
Q62: Which of the following salts is(are) considered
Q63: A famous brand of sweet and sour
Q64: Rank the following three acids in terms
Q66: What is the pH of an aqueous
Q67: The pH of a 1.00 liter solution
Q68: The pH of a vinegar solution is
Q69: Regarding acid strength, which of the following
Q70: Consider the salt pyridinium acetate, [(C<sub>5</sub>H<sub>5</sub>NH<sup>+</sup>)(C<sub>2</sub>H<sub>3</sub>O<sub>2</sub><sup>1 -