Multiple Choice
A 0.40 gram sample of NaOH (molar mass = 40.0 g/mol) was completely neutralized witH₆2.5 mL of H₂ SO₄solution. Assume the chemical equation is:
H₂ SO₄(aq) + 2 NaOH (aq) Na2 SO₄(aq) + 2 H₂ O ( 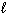 ) The molarity of the H₂ SO₄solution was:
) The molarity of the H₂ SO₄solution was:
A) 0.080 M
B) 0.16 M
C) 0.62 M
D) 6.4 M
E) 3.2 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q69: A sample of 100 mL of 0.10
Q70: What is the pH of an aqueous
Q71: A sample of 50.0 mL of 0.10
Q72: Exhibit 16-3 Consider the Titration curve below
Q73: A buffer was prepared by mixing 0.60
Q75: The titration of a weak acid, HA
Q76: Which of the following would not make
Q77: Calculate the pH of a solution made
Q78: A 25.0 mL portion of 0.0500 M
Q79: A buffer solution maintains a constant pH