Multiple Choice
Exhibit 16-1 Consider the equilibrium reaction below to answer the following question(s) . HNO₂ + H₂ O 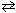 H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4
H₃O + + NO₂ 1 - K a (HNO₂ ) = 4.5 10 - 4
-Refer to Exhibit 16-1. What is the effect of adding NaNO₂ to an aqueous solution of HNO₂ as shown in the equilibrium reaction above?
A) The equilibrium will shift left favoring the reactant side and the pH will drop.
B) The equilibrium will shift right favoring the product side and the pH will drop.
C) There will be no shift in the equilibrium reaction and the pH will remain constant.
D) The equilibrium will shift left favoring the reactant side and the pH will rise.
E) The equilibrium will shift right favoring the product side and the pH will rise.
Correct Answer:

Verified
Correct Answer:
Verified
Q59: Exhibit 16-2 Consider titrating CH<sub>3</sub>COOH with standard
Q60: What is the pH of an aqueous
Q61: During a titration, 60.0 mL of 0.010
Q62: Which of the following is a polyprotic
Q63: What is the pH of a solution
Q65: What is the molarity of Ba(OH)<sub>2</sub> solution
Q66: In the titration of 0.100 M HCl
Q67: Consider the ionization of hydrofluoric acid as
Q68: Calculate the amount of ammonium chloride (NH<sub>4</sub>Cl)
Q69: A sample of 100 mL of 0.10