Multiple Choice
Which experiment listed below would provide a titration curve that resembles the titration shown below?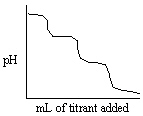
A) Titrating NH3 with standard NaOH (delivered from the burette) .
B) Titrating NH3 with standard HCl (delivered from the burette) .
C) Titrating H3PO4 with standard NaOH (delivered from the burette) .
D) Titrating Na3PO4 with standard HCl (delivered from the burette) .
E) Titrating Na3PO4 with standard NaOH (delivered from the burette) .
Correct Answer:

Verified
Correct Answer:
Verified
Q51: Oxalic acid is diprotic with p K
Q52: The following compounds have very limited solubility
Q53: Which of the following titration curves listed
Q54: Calculate the pH in a solution which
Q55: A solution of HCOOH ( K <sub>a</sub>
Q57: Consider a buffer solution containing a weak
Q58: What is the pH of an aqueous
Q59: Exhibit 16-2 Consider titrating CH<sub>3</sub>COOH with standard
Q60: What is the pH of an aqueous
Q61: During a titration, 60.0 mL of 0.010