Multiple Choice
Which of the following titration curves listed below best represents a curve for the complete titration of the weakly basic dianion, sulfide, S2 - , with a strong acid such as HCl as shown below in the net ionic equation?
S2 - (aq) + 2 HCl (aq) →H2S (g) + 2 Cl -
A) 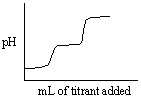
B) 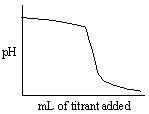
C) 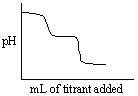
D) 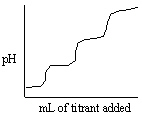
E) 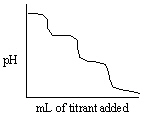
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: Which of the following titration curves listed
Q11: Exhibit 16-2 Consider titrating CH<sub>3</sub>COOH with standard
Q12: A 350 mL volume of 0.150 M
Q13: A buffer is made by dissolving 0.10
Q14: In the titration of 0.100 M HCl
Q16: Calculate the number of moles of sodium
Q17: Calculate the pH of a solution after
Q18: What is the pH of a buffer
Q19: Which pair(s) of substances would make a
Q20: What is the pH of a solution