Multiple Choice
Previously we indicated that the pH of a solution is defined in terms of logarithms as 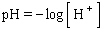 where
where 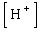 is the concentration of the hydrogen ion in that solution. Find the concentration of hydrogen ions in a glass of wine if the pH is 4.95 to three significant digits.
is the concentration of the hydrogen ion in that solution. Find the concentration of hydrogen ions in a glass of wine if the pH is 4.95 to three significant digits.
A) 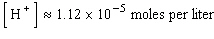
B) 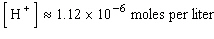
C) 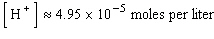
D) 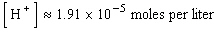
E) 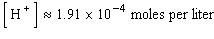
Correct Answer:

Verified
Correct Answer:
Verified
Q185: Solve the exponential equation. Round your answer
Q186: Solve the equation. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8808/.jpg" alt="Solve the
Q187: Find the magnitude M of an earthquake
Q188: Find a decimal approximation of the following
Q189: Find x . <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8808/.jpg" alt="Find x
Q191: Find the following logarithm to four decimal
Q192: Simplify the following expression. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8808/.jpg" alt="Simplify
Q193: In 1990, $699 billion were spent on
Q194: Use properties of logarithms to expand the
Q195: The function <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8808/.jpg" alt="The function