Multiple Choice
If we know the amount of light that is absorbed in a solution we can calculate the concentration of the substance dissolved in water using a spectrophotometer. For a certain substance the concentration (in moles/liter) is given by the formula  where
where 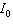 is the intensity of the incident light and
is the intensity of the incident light and 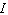 is the intensity of light that emerges. What is the concentration of the substance if the intensity
is the intensity of light that emerges. What is the concentration of the substance if the intensity 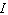 is 45% of
is 45% of 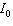 ?
?
A) 952 mol/L
B) -3667 mol/L
C) 2928 mol/L
D) 2192 mol/L
E) 1272 mol/L
Correct Answer:

Verified
Correct Answer:
Verified
Q189: If <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg" alt="If then
Q190: You calculate that your grade goes up
Q191: Solve the system of equations using the
Q192: The college Symphony Orchestra is performing and
Q193: Solve the system of equations using the
Q195: Two angles are supplementary. One angle has
Q196: Which one of the following exponential equations
Q197: Graphically determine the solution to the system
Q198: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg" alt=" " class="answers-bank-image d-inline" rel="preload"
Q199: For any <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg" alt="For any