Multiple Choice
Consider the following reaction. 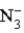 + H2O
+ H2O 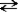 HN3 + OH− This reaction indicates that:
HN3 + OH− This reaction indicates that:
A) 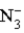 is a weak base.
is a weak base.
B) 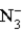 is a strong base.
is a strong base.
C) 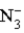 is a weak acid.
is a weak acid.
D) 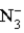 is a strong acid.
is a strong acid.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: Write the equation for the ionization of
Q26: The buffer solution in the flask was
Q27: Consider the image which depicts the reaction
Q31: When NaOH reacts with HCl, the net
Q40: When carbonated beverages are bottled or canned,
Q47: Which of the following substances can react
Q48: Consider the following three buffer reactions.<br>Buffer 1:
Q49: Which of the following will determine the
Q57: A solution composed of HCl and NaCl
Q64: A solution is 0.10 M in ascorbic