Multiple Choice
A buffer is composed of ammonia (NH3) and ammonium chloride (NH4Cl) . When base is added to the buffer, which of the following reactions occurs?
A) 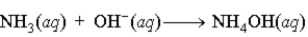
B) 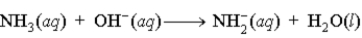
C) 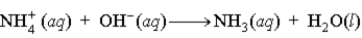
D) 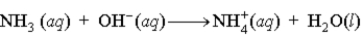
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Consider the following three buffer reactions.<br>Buffer 1:
Q7: What is the pH of a solution
Q11: What is the pH of a solution
Q19: Consider the following three buffer reactions.<br>Buffer 1:
Q38: Which of the following statements best describes
Q44: In converting from the pH of a
Q65: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1423/.jpg" alt=" Using the above
Q76: An ammonia solution has a pH of
Q78: Examine the structure given below <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8894/.jpg"
Q80: Adipic acid has the formula given below.