Multiple Choice
Consider the image which depicts the reaction between CH3COOH and H2O. Reactants:
Products: 
If the composition of the equilibrium mixture is as represented below 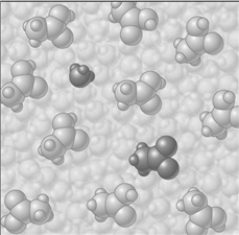
-A solution of KF in water will be
A) acidic.
B) basic.
C) neutral.
Correct Answer:

Verified
Correct Answer:
Verified
Q22: Consider the following three buffer reactions.<br>Buffer 1:
Q23: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1423/.jpg" alt=" Using the above
Q24: The molar concentration of H<sub>3</sub>O<sup>+</sup> in an
Q25: Which of the following is present in
Q26: The buffer solution in the flask was
Q28: Arterial blood gases (ABGs) were drawn on
Q29: All of the following solutions are 0.025
Q30: What is the pH of a 1
Q31: When NaOH reacts with HCl, the net
Q32: Consider the image which depicts the reaction