Short Answer
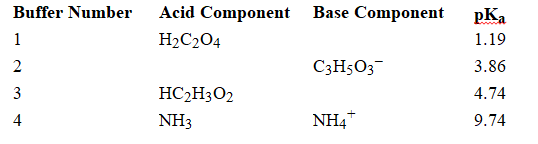 Using the above table, fill in the blank with the appropriate integer (1 ,2, 3,...) indicating the buffer and/or the letter indicating the formula below.
Using the above table, fill in the blank with the appropriate integer (1 ,2, 3,...) indicating the buffer and/or the letter indicating the formula below.
-Buffer ______________________ would be the most effective buffer in basic solutions.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Very rapid breathing can lead to a
Q6: The range of breaths per minute, also
Q8: In a solution of phosphoric acid all
Q10: Write the equation for the ionization of
Q33: Which of the following pH's corresponds to
Q40: When carbonated beverages are bottled or canned,
Q57: Below is the structure for the amino
Q71: Excess phosphorus is excreted by the kidneys.
Q72: Consider the following three buffer reactions.<br>Buffer 1:
Q78: The pK<sub>a</sub> of lactic acid is 3.86,