True/False
An element has the following electron arrangement. shell 1: 2 electrons shell 2: 8 electrons shell 3: 18 electrons shell 4: 18 electrons shell 5: 7 electrons
The Lewis structure for this element would be: 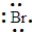
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Which of the following is the correct
Q13: Enter an integer number (1, 2, 3,
Q16: A sealed cylinder is filled with a
Q29: An element is a shiny gray solid
Q30: Which of the following correctly describes a
Q49: Enter an integer number (1, 2, 3,
Q58: An isotope of gallium consisting of 31
Q63: Based on the text periodic table and
Q70: Fill in each blank with the appropriate
Q75: Elements in the same period of the