Multiple Choice
Naturally occurring lithium (Li) consists of only two isotopes, Li-6 (6.02 amu) and Li-7 (7.02 amu) , where the exact isotopic masses are given in parentheses. Use the periodic table and determine which isotope is present in the larger percentage in the natural element.
A) 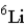
B) 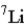
C) The percentage of each isotope is about the same.
D) The relative percent abundance cannot be determined from the information available.
Correct Answer:

Verified
Correct Answer:
Verified
Q19: Indicate the number of dots that should
Q23: Electron shells define a region in space
Q29: Consider the atomic view of a substance
Q35: In 22.99 g of Na there is
Q48: Based on the text periodic table and
Q51: Enter the chemical symbol of the element
Q59: What is the meaning of the subscripts
Q64: An elements with an electron arrangement of:<br>shell
Q67: Use the periodic table to determine about
Q78: Neutral isotopes of the same element have