Multiple Choice
A person drinks 1900 g of water, H2O, per day. How many moles of water did they consume?
A) 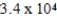 mol
mol
B) 0.009 mol
C) 105 mol
D) 18.02 mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: Indicate the number of dots that should
Q27: Enter the chemical symbol of the element
Q29: Consider the atomic view of a substance
Q36: The electron arrangement for Na would be:<br>shell
Q51: Enter the chemical symbol of the element
Q59: What is the meaning of the subscripts
Q60: Based on the text periodic table and
Q65: Sodium is a highly reactive metal and
Q73: The atomic weight of phosphorus (P) is
Q78: Neutral isotopes of the same element have