Multiple Choice
Which do you think has a higher boiling point, n-butanol or tert-butanol? 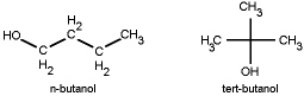
A) n-butanol because it forms stronger hydrogen bonds.
B) tert-butanol because it forms stronger hydrogen bonds.
C) n-butanol because the linear molecule allows for greater dispersion forces.
D) tert-butanol because the tetrahedral molecule allows for greater dispersion forces.
E) they will be exactly the same.
Correct Answer:

Verified
Correct Answer:
Verified
Q19: A liquid kinetically trapped below its freezing
Q20: Arrange the follow in order of increasing
Q21: A supercritical fluid has a density that<br>A)
Q22: Which do you think will have the
Q23: The triple point of a compound is
Q25: Generally if a liquid has stronger intermolecular
Q26: What types of intermolecular forces would you
Q27: The process of a solid converting directly
Q28: At the triple point<br>A) solid, liquid, and
Q29: In the phase diagram of water increasing