Multiple Choice
Refer to the figure.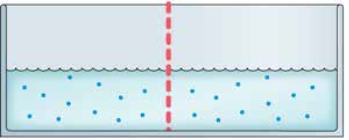 The two chambers in the figure are separated by a semipermeable membrane. What will happen to the fluid levels if salt is added to the left chamber?
The two chambers in the figure are separated by a semipermeable membrane. What will happen to the fluid levels if salt is added to the left chamber?
A) The solute concentration of the left chamber will increase, but the liquid levels will not change.
B) The difference in solute concentration will push salt and water molecules through the membrane until the concentrations are equivalent, which will overcome gravity and result in a higher level of fluid in the right chamber.
C) If enough salt is added, the difference in solute concentration will push salt molecules through the membrane until the osmotic pressure is less extreme, but the liquid levels will not change.
D) Water molecules from the right chamber will pass through the membrane until the ratio of water to salt is the same in both chambers, resulting in a higher level of fluid in the left chamber, despite gravity.
Correct Answer:

Verified
Correct Answer:
Verified
Q84: The primary source of energy for the
Q85: The main weakness of the previously accepted
Q86: One reason that body temperature is a
Q87: The hormone _, which induces drinking, is
Q88: Endotherms may show a significant, endogenously induced
Q90: Osmosensory neurons are cells that detect changes
Q91: Matching<br>-Isotonic<br>A) 0.9% NaCl<br>B) < 0.9% NaCl<br>C) NaCl<br>D)
Q92: Two physiological responses to hypovolemia are constriction
Q93: The response to an increase in cellular
Q94: Angiotensin II acts on the _ to