Multiple Choice
Boron trifluoride, BF3, is a corrosive material with a Lewis structure that shows boron to be two electrons short of a full octet. Which is the correct structure for BF3?
A) 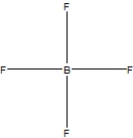
B) 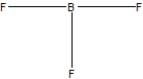
C) 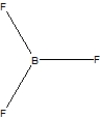
D) None of the above.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q113: How great is the electronegativity difference in
Q114: What is wrong with the statement, "nonpolar
Q115: How does Lewis theory explain the loss
Q116: What ketone can be smelled on the
Q117: How many linear or branched alkanes can
Q118: What does the double line, which looks
Q120: How is aniline best described?<br>A)As an alkene<br>B)As
Q121: What is the shape of the hydrogen
Q122: How are bioplastics made from corn starch
Q123: What is the octet rule?<br>A)Elements form bonds