Multiple Choice
Phosphine, PH3, is structurally much like ammonia. Which of the following is the best Lewis structure of phosphine?
A) 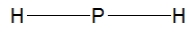
B) 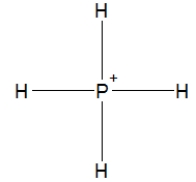
C) 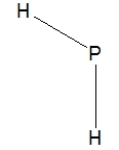
D) 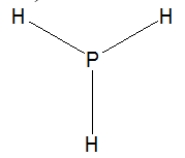
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: Draw the cyclic, organic molecule benzene, C<sub>6</sub>H<sub>6</sub>.
Q28: What geometry does the cisplatin molecule adopt?<br>A)Octahedral<br>B)Tetrahedral<br>C)Square
Q29: What can be the major limitation of
Q30: What is the shape of the hydrogen
Q31: What does VSEPR stand for?
Q33: How does the cocaine molecule interfere with
Q34: After drawing the Lewis structure for the
Q35: What trend do the noble gases display
Q36: What do the dots placed around an
Q37: What does the term "stereoisomer" indicate?<br>A)Two compounds