Multiple Choice
Boron trifluoride, BF3, is a corrosive material with a Lewis structure that shows boron to be two electrons short of a full octet. Which is the correct structure for BF3?
A) 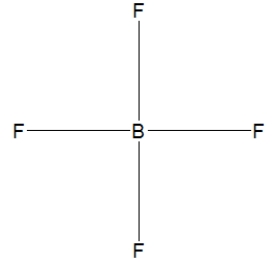
B) 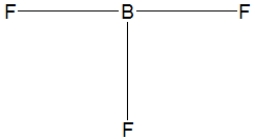
C) 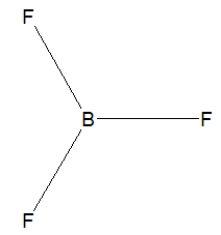
D) None of the above.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q77: The simplest covalently bonded molecule is hydrogen.
Q78: What is the best structural representation of
Q79: How many resonance structures can be drawn
Q80: What shape should the molecule SF<sub>4</sub> have?<br>A)A
Q81: What is the correct Lewis structure for
Q83: What is the geometry of the very
Q84: Draw for yourself the Lewis structure of
Q85: By what means do many illegal drugs
Q86: How is the N-Br bond described?<br>A)Pure covalent<br>B)Polar
Q87: What shape does the ammonia molecule have?<br>A)Trigonal