Multiple Choice
The concentration of a compound, X, is measured at different time intervals during the course of a chemical reaction. After plotting a graph of the concentration of X against time, shown in the graph below, the points labelled A-E on the graph have the following co-ordinates: A: x = 0 seconds; y = 0 mol L-1
B: x = 8 seconds; y = 0.37 mol L-1
C: x = 15 seconds; y = 0.49 mol L-1
D: x = 23 seconds; y = 0.63 mol L-1
E: x = 30 seconds; y = 0.62 mol L-1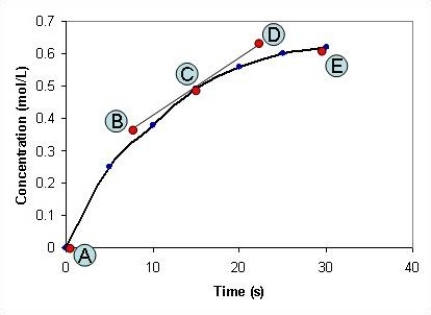 What is the rate of the chemical reaction after the reaction has been proceeding for fifteen seconds?
What is the rate of the chemical reaction after the reaction has been proceeding for fifteen seconds?
A) 3.26 mol L-1 s-1
B) 0.017 mol L-1 s-1
C) 0.021 mol L-1 s-1
D) 57.7 mol L-1 s-1
E) 48.39 mol L-1 s-1
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Which of the following statements concerning the
Q5: Which one of the following statements is
Q6: Match the type of enzyme inhibition with
Q7: When the temperature of a reaction is
Q8: The way in which the rate of
Q10: Which of the following correctly describes the
Q11: The activation energy of a reaction is
Q12: The following graph depicts the rate of
Q13: Look at the graph below. At which
Q14: An enzyme-catalyzed reaction can be represented by